Tutorial on the E1 (unimolecular elimination) alcohol dehydration reaction and mechanism, which converts alcohols into alkenes.
Related
Drawing Curved Arrows (introduction to drawing reaction mechanisms)
Carbocation Rearrangement Reactions (occur during alcohol dehydration reactions)
Hydration of Alkenes (reverse reaction of alcohol dehydration)
Reaction
In the presence of strong acids (such as sulfuric acid or phosphoric acid), secondary and tertiary alcohols can undergo a dehydration reaction via an E1 mechanism, converting the alcohol into an alkene:
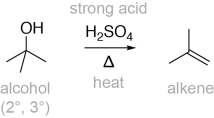
The term ‘dehydration’ is used to describe the loss of water from the starting material; an OH is removed from one carbon, and a H is removed from the other. This is actually the reverse of the alkene hydration reaction.
Under appropriate conditions (strong acid, 2° or 3° alcohol), alcohol dehydration can occur by an E1 (or unimolecular elimination) mechanism. Elimination describes the loss of two substituents from a molecule (-OH and –H), forming a double bond. Unimolecular describes the kinetics of the reaction. In an E1 mechanism, the rate determining step is carbocation formation (see mechanism below); this step depends only on one molecule, the alcohol. This differs from the E2 (or bimolecular elimination) mechanism, where the rate-determining step depends on two molecules (the substrate and base).
Depending on the structure of the alcohol, more than one alkene product may be possible. Zaitsev’s rule predicts that the most highly substituted alkene will be the major product of an E1 reaction (that is, the alkene with the most alkyl substituents on the alkene carbons). This is an example of regioselectivity, where the formation of a particular regioisomer is favoured.
As this reaction involves a carbocation intermediate, note that carbocation rearrangement reactions (e.g., 1,2-hydride and 1,2-alkyl shifts) are possible, and may result in unexpected products.
Mechanism
For an introduction to drawing reaction mechanisms, check out Drawing Curved Arrows (Part 1).
Let’s go through the E1 mechanism for this reaction:

When a strong acid (like sulfuric acid) is used as a reagent, check what parts of the molecule can get protonated (or, what functional groups can behave as bases). Usually, this will be an electron-rich group, mainly atoms with lone pairs, and π bonds. Here, the oxygen in the hydroxyl group has two lone pairs. Alcohols aren’t very basic, and are hard to protonate (unlike alkoxides, the conjugate bases of alcohols), but this is overcome by using strong acid and heat. So, in this mechanism, sulfuric acid protonates the alcohol, forming an oxonium ion:

As oxygen is strongly electronegative, it doesn’t like to be positively charged. As there is a strong drive to neutralize the charge on the oxygen, oxonium ions are strong acids (the reverse of the above step). Another way that the positive charge can be neutralized is by breaking the carbon-oxygen bond, which in this example, generates a 3° carbocation and releases a water molecule. Protonating the hydroxyl group has made it into a better leaving group – the neutral hydroxyl group is not a good leaving group. Alcohols can also be made into better leaving groups by converting them into sulfonate esters (such as tosylates and mesylates).

Remember the overall trend of carbocation stability: 3° > 2° > 1° > methyl. This is why the E1 dehydration mechanism usually happens only for secondary and tertiary alcohols. As this carbocation formation is the rate-determining step for this reaction, this is especially important.

In the final step, a base (in this example, a water molecule) deprotonates a carbon adjacent to the positively charged carbon, forming the carbon-carbon π bond, and neutralizing the positive charge on the carbon. However, there are three different carbons adjacent to the carbocation carbon – how do we know which one will be deprotonated? This is predicted by Zaitsev’s rule, which states that the most highly substituted alkene product will be the major product. So, we deprotonate the carbon that will give us the most substituted alkene possible (in green below):

The mechanism for this step is:

Note that carbocations are also good electrophiles, and can be directly attacked by a nucleophile, like in an SN1 nucleophilic substitution reaction. Therefore, the nucleophilic substitution mechanism may compete with the elimination mechanism, giving a mixture of products.
Summary
Secondary and tertiary alcohols can be converted into alkenes by treatment with strong acid (e.g., sulfuric acid, phosphoric acid):

This reaction can follow an E1 (unimolecular elimination) mechanism, which involves a carbocation intermediate. Protonation of the alcohol by the strong acid generates an oxonium ion, which acts as a leaving group, forming a carbocation. Deprotonation of a carbon next to the carbocation carbon generates the alkene product. Based on Zaitsev’s rule, the major product is usually the most highly substituted alkene (an example of regioselectivity).